Radioimmunoassay (RIA) is an in vitro nuclear medicine very sensitive technique used to measure concentrations of antigens (for example, hormone levels in the blood) without the need to use a bioassay.
Although the RIA technique is extremely sensitive and extremely specific, it requires specialized equipment, but remains the least expensive method to perform such tests. It requires special precautions and licensing, since radioactive substances are used. Today it has been supplanted by the ELISA method, where the antigen-antibody reaction is measured using colorimetric signals instead of a radioactive signal. However, because of its robustness, consistent results and low price per test , RIA methods are again becoming popular.
The RAST test (radioallergosorbent test) is an example of radioimmunoassay. It is used to detect the causative allergen for an allergy.
To perform a radioimmunoassay, a known quantity of an antigen is made radioactive, frequently by labeling it with gamma-radioactive isotopes of iodine attached to tyrosine. This radiolabeled antigen is then mixed with a known amount of antibody for that antigen, and as a result, the two chemically bind to one another. Then, a sample of serum from a patient containing an unknown quantity of that same antigen is added. This causes the unlabeled (or "cold") antigen from the serum to compete with the radiolabeled antigen ("hot") for antibody binding sites.
As the concentration of "cold" antigen is increased, more of it binds to the antibody, displacing the radiolabeled variant, and reducing the ratio of antibody-bound radiolabeled antigen to free radiolabeled antigen. The bound antigens are then separated from the unbound ones, and the radioactivity of the free antigen remaining in the supernatant is measured using a gamma counter. Using known standards, a binding curve can then be generated which allows the amount of antigen in the patient's serum to be derived.
History
It was developed by Rosalyn Yalow and Solomon Aaron Berson in the 1950s. In 1977, Rosalyn Sussman Yalow received the Nobel Prize in Medicine for the development of the RIA for insulin: the precise measurement of minute amounts of such a hormone was considered a breakthrough in endocrinology.
With this technique, separating bound from unbound antigen is crucial. Initially, the method of separation employed was the use of a second "anti-antibody" directed against the first for precipitation and centrifugation. The use of charcoal suspension for precipitation was extended but replaced later by Drs. Werner and Acebedo at Columbia University for RIA of T3 and T4.
An ultramicro RIA for human TSH was published in BBRC (1975) by Drs. Acebedo, Hayek et al.
Radioimmunoassay
Radioimmunoassay
The technique of radioimmunoassay has revolutionized research and clinical practice in many areas, e.g.,
- blood banking
- diagnosis of allergies
- endocrinology
The technique was introduced in 1960 by Berson and Yalow as an assay for the concentration of insulin in plasma. It represented the first time that hormone levels in the blood could be detected by an in vitro assay.
The Technique
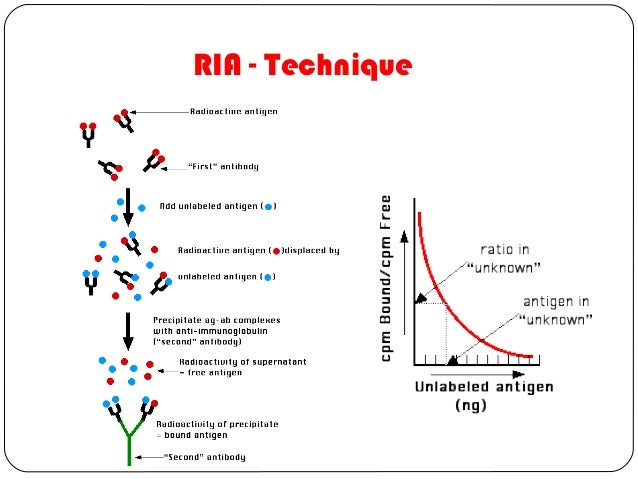
- A mixture is prepared of
- radioactive antigen
- Because of the ease with which iodine atoms can be introduced into tyrosine residues in a protein, the radioactive isotopes 125I or 131I are often used.
- antibodies against that antigen.
- Known amounts of unlabeled ("cold") antigen are added to samples of the mixture. These compete for the binding sites of the antibodies.
- At increasing concentrations of unlabeled antigen, an increasing amount of radioactive antigen is displaced from the antibody molecules.
- The antibody-bound antigen is separated from the free antigen in the supernatant fluid, and
- the radioactivity of each is measured.
- From these data, a standard binding curve, like this one shown in red, can be drawn.
- The samples to be assayed (the unknowns) are run in parallel.
- After determining the ratio of bound to free antigen in each unknown, the antigen concentrations can be read directly from the standard curve (as shown above).
Separating Bound from Free Antigen
There are several ways of doing this.
- Precipitate the antigen-antibody complexes by adding a "second" antibody directed against the first. For example, if a rabbit IgG is used to bind the antigen, the complex can be precipitated by adding an antirabbit-IgG antiserum (e.g., raised by immunizing a goat with rabbit IgG). This is the method shown in the diagram above.
- The antigen-specific antibodies can be coupled to the inner walls of a test tube. After incubation,
- the contents ("free") are removed;
- the tube is washed ("bound"), and
- the radioactive of both is measured.
- The antigen-specific antibodies can be coupled to particles, like Sephadex. Centrifugation of the reaction mixture separates
- the bound counts (in the pellet) from
- the free counts in the supernatant fluid.
Radioimmunoassay is widely-used because of its great sensitivity. Using antibodies of high affinity (K0 = 108–1011 M−1), it is possible to detect a few picograms (10−12 g) of antigen in the tube.
The greater the specificity of the antiserum, the greater the specificity of the assay.
Both 125I or 131I emit gamma radiation that requires special counting equipment;The main drawbacks to radioimmunoassay are the expense and hazards if preparing and handling the radioactive antigen.
- The body concentrates iodine atoms — radioactive or not — in the thyroid gland where they are incorporated in thyroxine (T4).
Despite these drawbacks, RIA has become a major tool in the clinical laboratory where it is used to assay
- plasma levels of:
- most of our hormones;
- digitoxin or digoxin in patients receiving these drugs;
- certain abused drugs
No comments:
Post a Comment